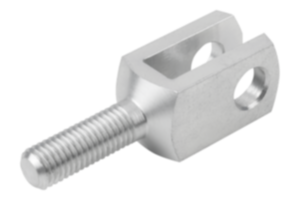
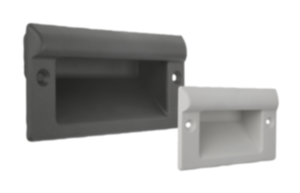
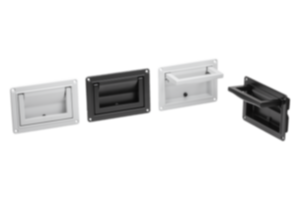
Operating principle and properties of KIPP MEDIgrip
Microorganisms such as germs, bacteria or fungi that contaminate surfaces and operating parts, can multiply many times over within a few hours under certain climatic conditions. KIPP MEDIgrip products use microsilver technology to prevent this propagation. Tiny silver particles (microsilver) are already incorporated into the plastic granulate used for the operating parts. The microsilver constantly releases silver ions onto the component surface. Vital functions of microorganisms adhering there, including multi-resistant germs, are impaired by the silver ions, which leads to the demise of these organisms. A further spreading of the microorganisms is prevented and the existing germs are completely eliminated in a few hours.
For MEDIgrip, KIPP uses a plastic (polyamide) with special antimicrobial properties. The products of other manufacturers are often only surface-treated. The disadvantage of this is that silver particles are removed through wear. With MEDIgrip, longevity is already ensured during the manufacturing process. The entire operating parts are permeated with silver particles already contained within the plastic. This means that they retain their full antimicrobial effect even with light abrasion and can be used for several years.
Scientifically proven effectiveness
KIPP has had the MEDIgrip product line tested for its effectiveness in extensive trials by a certified medical laboratory. Within the framework of a kinetics test as well as two internationally recognised ASTM tests, a bacterial reduction of at least 99.9 % was successfully demonstrated with the following test microbes:
- Pseudomonas aeruginosa (widespread and highly resistant hospital pathogen)
- Staphylococcus aureus (multi-resistant bacteria MRSA, also partially resistant to antibiotics)
The effectiveness of the microsilver technology was previously proven in tests with other bacteria. The knowledge gained can be transferred to the MEDIgrip product line and so, also confirm the effectiveness for the following bacteria:
- Staphylococcus aureus
- Staphylococcus epidermidis
- Multi-resistant Staphylococcus aureus (MRSA)
- Multi-resistant Staphylococcus epidermidis (MRSE)
- Vancomycin Resistant Enterococcus (VRE)
- Escherichia coli
- Pseudomonas aeruginosa (normal und multiresistent)
- Enterococcus faecalis
- Enterococcus faecium
- Listeria monocytogenes
- Acinetobacter baumannii
- Salmonella choleraesuis
- Klebsiella pneumoniae
- Proteus mirabilis
- Streptococcus mutans
- Streptococcus bovis
- Streptococcus intermedius
- Streptococcus sanguinis
- Streptococcus oralis
- Streptococcus gordonii
- Citrobacter freundii
- Corynebacterium striatum
- Corynebacterium xerosis
Certified skin tolerance – Successful testing for biocompatibility
Special large size microsilver particles (not nano-silver) are used in the products. This does not cause any adverse health effects for the user. The skin compatibility or biocompatibility of MEDIgrip was also successfully tested and confirmed according to ISO 10993-5 (tests for in vitro cytotoxicity) in an accredited laboratory.
Application sectors
Due to the resistance to moisture and cleaning agents, the excellent long-term effect and effectiveness against multi-resistant bacteria, MEDIgrip from KIPP finds use in many different sectors and applications:
- Medical facilities and clinics
- Medical equipment
- Doctors surgeries
- Retirement and nursing homes
- Fitness studios and rehabilitation clinics
- Foodstuff industries
- Beverage bottling plants
- Public areas and public transport
- Operational facilities
- Commercial canteens and sanitary areas